
The primary activities of Stites & Associates, LLC, (SALLC), a technology development and improvement company working in a wide variety of energy applications, include: setting up labs and experiments, evaluating data, evaluating lab operations, and evaluating and improving technologies. Ron Stites, managing member of SALLC, was formerly the director of research for Range Fuels and holds several three patents in alternative fuels. SALLC has a research laboratory in Brighton, Colorado that performs gas chromatography/mass spectroscopy (GC/MS), Cyclic Voltammetry and Optical Microscopy. Stites believes that combining excellent analytical work with diligent research and “outside-the-box” thinking makes it possible for the company to evaluate existing technology as well as gain insight into the best ways to try to improve a technology. “Often this combination results in learning how to make non-obvious improvements that can result in real breakthroughs,” says Stites.
In this application, a supplier of industrial equipment wanted to market an existing product to companies producing ethanol from corn. There was anecdotal evidence indicating that the device increased ethanol yield. However, prior to marketing the device the company wanted to find the best operating conditions and determine what performance ethanol producers could expect from the product. The industrial equipment supplier contracted with SALLC to develop a method for measuring how well the supplier’s product improved performance to the corn ethanol market.
The first steps were determining the right measurement method and finding a way to preserve samples in order to prevent their deterioration during shipment from plant to lab. After an accurate measurement method had been identified, the next step was running a number of tests to gain insight into what factors affected sample preservation. Finally, researchers performed a design of experiments (DOE) that quantified the effects of these factors, singly and in combination, on sample preservation. The sample preservation method identified by the DOE has to date functioned well on hundreds of samples from ethanol plants across the Midwest.
Producing Ethanol from Corn
The process of producing ethanol from corn begins with soaking the grain in water. This makes it easier to separate the components on the corn kernel. The corn is then milled to produce a corn flour that is slurried with water and a heat-stable enzyme – α-amylase – is added. This slurry is cooked in a process known as liquefaction. which uses heat and mechanical shear to break apart starch molecules into small fractions. After liquefaction the corn slurry, also known as corn mash, is cooled to approximately 30oC and glucoamylase enzyme is added to complete the breakdown of starch into glucose. In the fermentation step, yeast is added to the corn mash to convert the simple sugars to ethanol.
The breakdown of starch to glucose takes place in two steps, from starch to maltrodextrins and then from maltodextrins to glucose. The equipment supplier hypothesized that its product could hasten the breakdown of starch to glucose while also using less enzymes. The goal was also to minimize “burning,” a phenomenon that occurs when shorter-chain sugars react together and with proteins to form polymers that yeast cannot digest. In fact, some of the polymers are even toxic to yeast. This process is monitored by analysis of the degree of polymerization (DP) of the maltodextrins. But measuring maltrodextrins is challenging. The key difficulty is that the analysis requires specialized techniques that are not available in the plant so samples must be sent to the lab. But the samples are not stable so the breakdown of starch continues during transport.
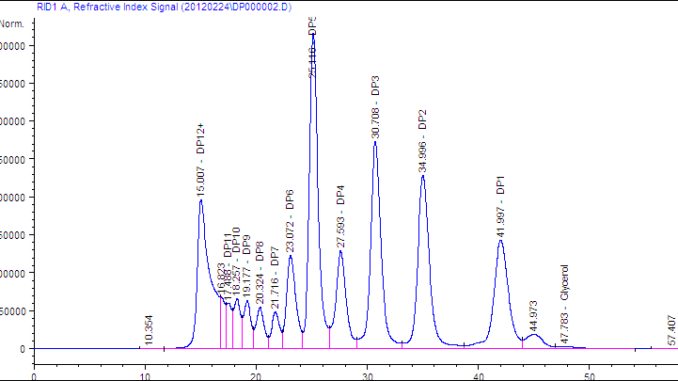
Figure 1: DP measurements of maltodextrin (HPLC results)
Increasing Sample Preservation Time
There are roughly 200 ethanol plants in the U.S. Midwest and samples would need to be shipped to the SALLC lab from any plant that tried the new product. Stites recognized that he needed to find a way to stop the reaction for 72 hours, approximately the time it would take for a sample to be shipped to the lab and tested. Samples from the actual production process were too inconsistent to be used for creating a sample preservation process. Stites found that spray-dried maltodextrin provides a consistent starting material to evaluate methods of stopping the reaction. Researching the enzymes, he found that they did not work well at low pH and low temperatures. So he lowered the pH by adding sulfuric acid, cooled the samples and held them at shipping times from 24 to 72 hours before testing. A pH of 2 appeared to prevent the enzymes from working. Stites reviewed published literature and found a new method based on high performance liquid chromatography (HPLC) that could accurately measure maltodextrins. He set up equipment in the SALLC lab to perform the method. The HPLC method easily detected changes in sugar concentration (see Figure 1).
Once this preliminary work was completed, the actual DOE was relatively simple. Earlier, while working at Range Fuels, Stites had begun using Design-Expert® software from Stat-Ease, Inc., Minneapolis, Minnesota, after a colleague at the company spoke highly of the program. Stites received training from Stat-Ease and began using the software at Range. ”I am not a statistician, I am an experimenter,” Stites says. “Design-Expert fits my needs because it is designed for use by subject matter experts who are not necessarily experts in statistical methods. The software walks users through the process of designing and running the experiment and evaluating the results. Stat-Ease also provides very good support. I contact them not only for questions about using the software, but also to check out my statistical thinking and they have always been very helpful. I love working with them.”
Table 1: Factors and results from DOE used to explore sample preservation methods
DOE Methods and Results
The factors, runs and results for the preservation DOE are shown above in Table 1. The %<DP9 in the sample at the beginning of the experiment is 25.00%. The response %<DP9 shows how much this value increased during the experiment. The results show that the most important factor is pH. All of the other factors had minimal effects, although incubation at 60oC made things slightly worse by speeding up the reaction. The best results were observed when pH was adjusted to 2.0 and storage was maintained at 3oC. With this method, the DP increased to only 25.04% after 48 hours. This is less than the standard deviation of the HPLC method and this method can be easily performed in the field.
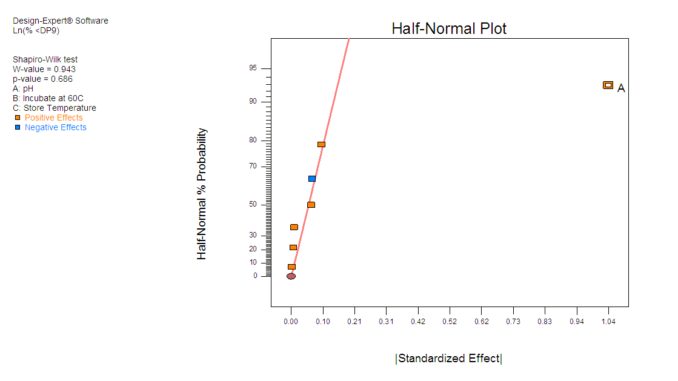
Figure 2: As shown in this half-normal plot, DOE proved the pH results to be significant
Next Stites needed to determine whether or not these results are significant or if they could have been achieved by chance. Factorial design analysis uses the half-normal plot to identify significant effects. The orange and blue rectangles on the half-normal plot in Figure 2 show the effects, positive and negative respectively, and the position of these rectangles reflect the relative size. The further the factor effects are from the line near zero, the more likely they are to be significant. In this case, the factor effects of variable A, which is pH, are significantly greater than the variation between the insignificant effects, demonstrating the statistical significance of the experiment.
The method used for run 6 was adopted and worked very well from the beginning for samples from the slurry and liquefaction steps. Some strange results occurred with the fermentation samples – most of the carbohydrates had disappeared. Stites discovered that the sulfuric acid was interfering with the HPLC measurements. The method was modified to remove the sulfuric acid before analysis by treatment with barium hydroxide and filtering. Since this modification was made, the method has successfully worked with samples from all three steps.
Ethanol plants from throughout the Midwest shipped hundreds of samples, which were then used to evaluate the performance of the new product. Some plants found significantly better results and others did not see significant improvements. “The DOE was clearly successful in its ability to identify and validate a measurement method that enabled us to accurately evaluate the performance of a new product in a large number of plants under a wide range of operating conditions,” says Stites.
For more information, contact:

Jerry Fireman is a technology writer who specializes in writing about computer aided design (CAD), 3D printing, computer-aided engineering (CAE), the Internet of Things (IoT), electronic engineering, pharmaceutical research and manufacturing, test and measurement and a variety of other topics.
–Stites & Associates, LLC, Ron Stites, Managing Member, 449 Mount Sherman, Brighton, CO, 80601. Ph: 912-247-6120, E-mail: [email protected] , Website: http://www.tek-dev.net , Blog: http://tek-dev.typepad.com/technology-development/
–Stat-Ease, Inc., 2021 E. Hennepin Avenue, Ste. 480, Minneapolis, MN 55413-2726. Ph: 612-378-9449, Fax: 612-746-2069, E-mail: [email protected], Web site: http://www.statease.com


Leave a Reply